|
JAK2 Inhibitor Therapies for the Frontline Setting
Currently, there are only two JAK2 inhibitor agents that have been approved for treatment of MF. These include ruxolitinib, which was approved in 2011; and fedratinib, which was approved in 2019. There are other novel JAK2 inhibitor therapies are under investigation (Table 1). This section will review the available therapies and those that are further along in development.
Table 1. Currently available and emerging JAK2 inhibitors for myelofibrosis.1
Drug |
Other Target |
Phase |
Status |
Ruxolitinib |
JAK-1 |
III |
Approved |
Fedratinib |
FLT-3, RET |
III |
Approved |
Pacritinib |
FLT-3 |
III |
Ongoing |
Momelotinib |
JAK1, JNK1, TYK2, CDK2, RICJ2 |
III |
Ongoing |
NS-018 |
SRC, FLT3, ABL |
I/II |
Ongoing |
Ruxolitinib
Ruxolitinib is a selective JAK1/2 inhibitor that was approved based on findings from the pivotal COMFORT-I and -II trials, in which the agent significantly improved spleen response and total symptom score (TSS) vs placebo2 and best available therapy (BAT)3 (View Table 2 here).
Findings from an analysis of 5-year pooled data from the COMFORT trials demonstrated continued efficacy with ruxolitinib, which reduced the risk of death by 30% vs comparators (median OS, 5.3 vs 3.8 years, respectively; P=.0065).7 This OS benefit was especially more evident among patients who were originally randomized to receive ruxolitinib vs those who crossed over from the control group (median OS, 5.3 vs 2.3 years). Ruxolitinib was also observed to extend OS vs controls in an analysis of OS censoring patients at the time of crossover (median OS, 5.3 vs 2.4 years, respectively; P=.0013). Finally, ruxolitinib provided an OS benefit regardless of anemia status at baseline or transfusion requirements at week 24. The survival benefit is derived mostly from improvement in patient symptoms and performance status rather true disease altering effect.
Fedratinib
Fedratinib was approved in August 2019 for patients with intermediate-2 or high-risk primary or secondary (post-polycythemia vera [PV] or post-essential thrombocythemia [EV]) MF. This agent selectively inhibits JAK2/FLT and also RET and was approved based on findings from the JAKARTA-1 and -2 trials.4,8 Notably, these studies included patients with platelet counts of ≥50 x 109/L, which is lower than the inclusion criterion of ≥100 x 109/L in the ruxolitinib COMFORT trials.2,3
JAKARTA-1 was a 3-arm trial in which patients (N=289) were randomly assigned to receive fedratinib at a dose of either 400 mg or 500 mg; or placebo.4 At both doses, fedratinib significantly reduced spleen volume vs placebo at 24 weeks and confirmed 4 weeks later (View Table 2 here). Thirty-six of patients in the fedratinib 400 mg group and 40% in the 500 mg group met the primary endpoint, compared with 1% of those who received placebo (P<.001 for comparisons of both doses vs placebo). In addition to these patients, an additional five patients in each fedratinib treatment arm achieved splenic responses of at least 35% at 24 weeks but were not confirmed 4 weeks later. JAK2 mutational status, MF disease subtype, and risk status did not affect spleen responses.
Pacritinib
Pacritinib is a selective inhibitor of JAK2/FLT-3 that was studied in a phase 3 PERSIST-1 trial vs BAT.5 Similar to the COMFORT and JAKARTA series, PERSIST-1 was conducted among patients with intermediate-2 and high-risk MF; there was no platelet threshold or cut off to enroll on the study. Patients received pacritinib 400 mg once daily, and results showed an overall spleen response of 25%. Among those with platelets <100 x 109/L, spleen response was 24% and in patients with platelets <50 x 109/L, spleen responses were observed in 33% (View Table 2 here). Overall, the TSS reduction was 19%. Anemia and thrombocytopenia were observed in 17% and 21%, respectively, of patients, similar what was observed with fedratinib.
Momelotinib
The phase 3 SIMPLIFY-1 trial evaluated momelotinib, a potent and selective inhibitor of JAK1/2, vs ruxolitinib in ruxolitinib-naïve patients with high-risk or intermediate-2 MF.9 Patients were randomly assigned to receive either momelotinib 200 mg once daily or ruxolitinib 20 mg twice daily. Efficacy results showed that momelotinib was noninferior to ruxolitinib in regard to spleen volume reduction (26.5% vs 29%, respectively; P=.11), however, noninferiority was not met regarding TSS (28.4% vs 42.2%, respectively, P=.98. Momelotinib improved red blood transfusion rate.
The Potential Role of the Available and Emerging JAK2 inhibitors in the Treatment of MF
Based on the available data, ruxolitinib remains in majority of the cases the current standard of care for patients with intermediate- and high-risk MF with platelets of >100 x 109/L. Fedratinib has elicited similar spleen responses in the same patient population, with perhaps less symptom reduction. Fedratinib was associated with higher rates of GI toxicity vs ruxolitinib in their respective studies, which is probably an effect of the FLT3 inhibitory activity of fedratinib. Fedratinib also was elicited less myelosuppression vs ruxolitinib. However, comparing fedratinib vs ruxolitinib is difficult since there have not been any head-to-head trials comparing the two agents, and these observations are based solely on the findings from their respective trials. There could be a role for fedratinib in upfront therapy in patients not tolerant to ruxolitinib, especially with borderline cytopenias.
Regarding the investigational agents, currently available data indicated that pacritinib may be the treatment choice for patients with severe thrombocytopenia, whereas momelotinib may become the preferred therapy for those with anemia.
Improving Outcomes with Ruxolitinib: Investigational Combination Therapies
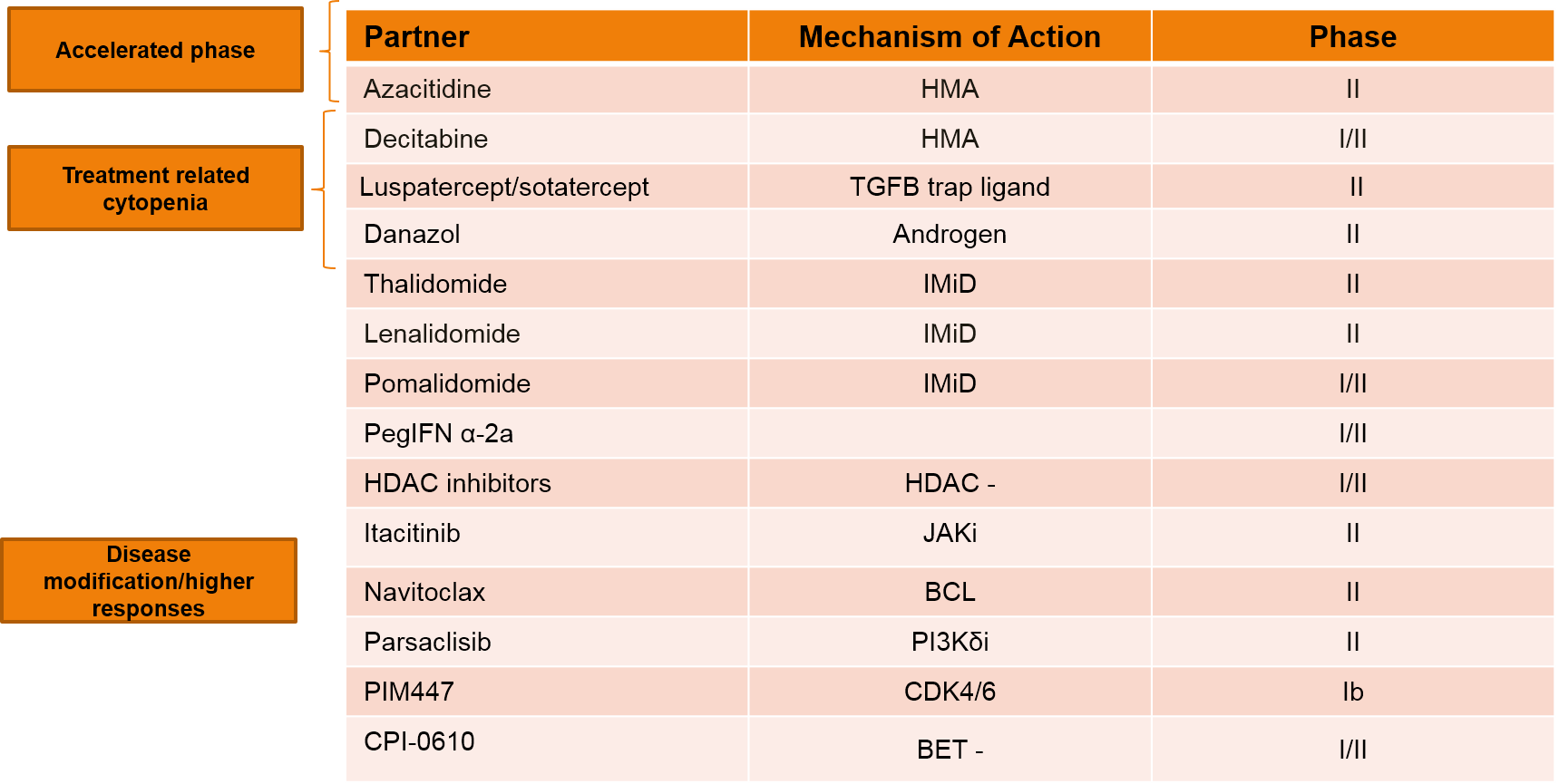
Currently, there are multiple studies evaluating various ruxolitinib combination strategies in the frontline setting, with the goal of improving responses or the durability of response. Table 3 shows some of the combination strategies that are currently under investigation. In general, it can be said that the combination of ruxolitinib with hypomethylating agents is under investigation primarily in patients with accelerated phase disease, with the goal of improving symptoms. Combination with other agents such as luspatercept and sotatercept, are being studied to alleviate anemia. Thalidomide with ruxolitinib is being studied to improve platelet and anemia responses, whereas other agents such as lenalidomide, interferon, navitoclax, among others, are believed to modify the disease and/or improve responses.
Table 3. Ruxolitinib-based Combination Therapies Under Investigation.
Ruxolitinib Failure
Outcomes after ruxolitinib failure are very poor, and there remains a substantial unmet clinical need to develop new treatment approaches in this setting. Patients who fail ruxolitinib generally either develop accelerated-phase disease or severe cytopenias. Many patients also experience recurrence of spleen symptoms or constitutional symptoms. Findings from one clinical trial showed that 35% of patients with MF died while receiving ruxolitinib therapy, and that the median survival after discontinuing therapy was 14 months.10 Factors that were associated with shorter survival included platelet counts of <260 × 109/L at the start of therapy or <100 × 109/L at the time of discontinuation and clonal evolution during therapy. Similar survival outcomes were observed in a study conducted in a real-world setting; in this study, the most common reason for discontinuing ruxolitinib therapy was the development of cytopenias, and the median OS was 13 months.11
The traditional definition of response is presented in Table 4.12 This was developed by the International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and is used in clinical trials to define response.
Table 4. Revised International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet Response Criteria for Myelofibrosis.12
|
However, due to the development of novel agents, the definition of ruxolitinib failure has been proposed to describe and differentiate refractory, relapsed, and intolerant disease, and to provide a description of suboptimal response to ruxolitinib therapy (Figure 1).13 This is important in the context of the development of new agents for the post-ruxolitinib setting, where there were no therapies, and patient would just discontinue ruxolitinib.
Current and Emerging Novel JAK2 Approaches in the Second-line Setting
Fedratinib
The single-arm phase 2 JAKARTA-2 study evaluated fedratinib in patients with ruxolitinib-resistant or intolerant, high-risk primary or secondary MF.8 In this trial, fedratinib was given at a dose of 400 mg once daily, for six consecutive 4-week cycles. The primary endpoint was spleen response (≥35% reduction in spleen volume as determined by CT and MRI), which was achieved by 55% of the 83 assessable patients. The most common grade 3-4 AEs were anemia and thrombocytopenia, with no reports of encephalopathy as was observed in the JAKARTA-1 trial.
A recent re-analysis of JAKARTA-2 was conducted to apply the more stringent definitions of ruxolitinib resistance and intolerance (Figure 1) to the overall study population.13 In this analysis, 79 of the 97 enrolled patients met the new criteria for ruxolitinib resistance (n=62; relapsed: n=18; refractory: n=47) and intolerance (n=14). The results showed that rates of SVR were 28%, 31%, and 29% in patients who had disease that was relapsed, refractory, and intolerant to ruxolitinib, respectively. Toxicities were consistent with prior reports, with no new safety signals identified. In addition, recent findings indicate that fedratinib elicited robust spleen response rates, regardless of the reason for ruxolitinib discontinuation.14,15 An analysis of HRQoL benefit showed that fedratinib provided clinically meaningful improvement across patients subgroups.16,17
Figure 1. Updated definitions of ruxolitinib failure13
|
Pacritinib
The phase 3 PERSIST-2 trial evaluated pacritinib at two doses, 400 mg once daily and 200 mg twice daily, vs best available therapy (BAT), including ruxolitinib.6 Patients who received prior treatment with one or two JAK inhibitors were allowed for enrollment. Results showed that more patients receiving pacritinib 200 mg had higher rates of spleen response and reduction in TSS vs those on the 400-mg dose or on BAT (Table 5).
Table 5. Efficacy Findings Among Patients with Prior Ruxolitinib in the PERSIST-2 Trial6
Measure |
Pacritinib 400 mg |
Pacritinib 200 mg |
BAT |
| Patients with ≥35% SVR | |||
Achieved end point, n (%) |
2 (6) |
4 (13) |
1 (3) |
95% CI for the %* |
0.8-21.4 |
3.6-29.8 |
0.1-15.8 |
| Patients with ≥35% SVR | |||
Achieved end point, n (%) |
3 (10) |
10 (32) |
5 (15) |
95% CI for the %* |
2.0-25.8 |
16.7-51.4 |
5.1-31.9 |
BAT=best available therapy; CI=confidence interval; SVR=spleen volume reduction; TSS=tumor symptoms score
Momelotinib
The phase 3 SIMPLIFY-2 trial was designed to test the superiority of momelotinib vs BAT in regard to spleen response (≥35% spleen volume at 24 weeks in patients with primary myelofibrosis (PMF) or post-PV/ET myelofibrosis who received prior ruxolitinib for at least 28 days.18 Patients had to have had either suboptimal responses to ruxolitinib or hematological toxic effects with ruxolitinib therapy. Patients were randomly assigned in a 2:1 fashion to receive momelotinib 200 mg once daily (n=104) vs BAT (n=52), which could include ruxolitinib, chemotherapy, steroid, no treatment, or other standard interventions. Results showed similar rates of SVR between treatment arms (momelotinib: 7%, BAT: 6%), and the study thus did not meet the primary endpoint. Rates of grade ≥3 anemia and thrombocytopenia were similar between treatment arms, but there were fewer reports of abdominal pain in the momelotinib group vs BAT (1% vs 6%, respectively).
Beyond JAK Inhibition: Novel Therapies in the Second-line Setting
As mentioned above, outcomes for patients following ruxolitinib failure are dismal, and new therapies and treatment strategies are urgently needed in this setting. Table 6 summarizes the novel therapies that are under investigation in this setting. The following section will review some of the most compelling data from these trials.
Table 6. Novel Therapies Under Investigation in the Second-line Setting of Myelofibrosis19
| Target | Agent | |
| Promotion of apoptosis | SMAC mimetic/IAP BCL-xL inhibitors LSD-1 inhibitors XPO1 inhibitors |
LCL-161 Navitoclax IMG-728 Selinexor |
| Targeting hematopoietic stem cell/ microenvironment | CD123 Hsp90 |
Tagraxofusp PU-H71 |
| Modulation of TP53 pathway | MDM2 antagonists | Idasanutlin KRT-232 |
| Targeting fibrosis and associated cytokine | Pentraxin-2 | PRM-151 |
| Aurora kinase inhibition | -- | Alisertib |
| Telomerase inhibition | -- | Imetelstat |
| Bromodomain and extraterminal protein inhibition | BET | CPI-0610 |
Imetelstat
Imetelstat is a covalently lipidated 13-mer oligonucleotide that binds to human telomerase RNA and has been shown to be a potent competitive inhibitor of telomerase activity.20,21 Imetelstat has been evaluated a phase 2 trial that tested the agent at two dose levels, 4.7 mg/kg q 3 weeks (n=48) and 9.4 mg/kg q 3 weeks (n= 59), in patients with intermediate- or high-risk MF that progressed during or after JAK-inhibitor therapy and who had active symptoms of disease.22 Among these patients, the median time on JAK inhibitor therapy was 23 months, and the median platelet count was 147 x 109/L. About a quarter of patients had triple-negative disease (ie, no mutations of JAK2, MPL, or CALR), and 67% had high-molecular risk disease (≥1 mutation of ASXL1, EZH2, SRSF2, or IDH1/2).
At clinical cut off, the median follow-up was about 23 months, and the median duration of treatment was 6 months.22 At week 24, 6 patients in the 9.4 mg/kg arm had a ≥35% SVR vs 0 in the 4.7 mg/kg group; moreover, ≥10% SVR was reported in 23 patients at the higher dose. More patients receiving the higher dose also had higher TSS reduction at week 24 vs those on the lower dose (19 vs 3 patients, respectively). Importantly, median OS was about 30 months in the 9.4 mg/kg arm vs 20 months in the 4.7 mg/kg arm, suggesting a signal of improved survival with imetelstat.
A case-control study that evaluated imetelstat vs BAT in a cohort of patients similar to those included in the IMBARK study from Moffitt Cancer Center.23 Data showed that imetelstat conferred a 65-67% lower risk of death vs BAT, supporting the previous findings that the agent provides favorable OS compared to historical real-world.
PRM-151
PRM-151 is a recombinant human pentraxin-2 molecule that has been shown to prevent and reverse fibrosis in animal models of MF.24 This agent was studied at three dose levels (0.3 mg/kg, 3 mg/kg, and 10 mg/kg) for at least 9 cycles in a randomized phase 2 trial in 97 patients with advanced MF.25 PRM-151 resulted in bone marrow fibrosis at all dose levels (30%, 28%, and 25%, respectively) at any time point, and also increases in hemoglobin and platelet count or reduction in transfusion requirements. About a third of patients experienced a decrease in bone marrow collagen grade, and PRM-151 also elicited reduction in spleen volume, with a maximum reduction of 34% in one patient.
CPI-0610
The brodomain and extraterminal domain inhibitor (BETi), CPI-0610, was evaluated as either monotherapy (Arm 1) or “add-on” therapy to ruxolitinib (Arm 2) in the open-label, phase 2 MANIFEST trial.26 Each treatment arm was further stratified based on transfusion dependency. Arm 1 included patients were no longer on ruxolitinib, whereas Arm 2 consisted of those who had a suboptimal response to ruxolitinib or disease progression. This trial included patients with ruxolitinib-refractory or -intolerant advanced MF. CPI-0610 was given at a dose of 125 mg QD, 2 weeks on, 1 week off, in a 21-day cycle; the dose could be increased to up to 225 mg QD only in Arm 1.
Recently presented data showed that CPI-0610, as either monotherapy or add-on therapy, is safe and generally well tolerated, with the potential for meaningful disease modification.26 Among transfusion-dependent patients who received CPI-0610 as add-on therapy, 25% had a ≥35% reduction in spleen volume, with a median change of -24.9%, and a ≥50% response in TSS observed in 54%. Moreover, 6 of 14 patients who were transfusion dependent became transfusion independent.
Navitoclax
Navitoclax was investigated in a phase 2, single-arm open-label trial in combination with ruxolitinib in patients with primary or secondary MF.27 Patients had to had received at least 12 weeks of continuous ruxolitinib therapy before starting navitoclax, which was given at a starting dose of 50 mg once daily. Dose-escalation of navitoclax was allowed based on platelet count and tolerability to a maximum dose of 300 mg.
Results showed that 23 of the 34 patients enrolled on the study achieved the 300 mg dose of navitoclax, and 22 of 25 patients who enrolled on ruxolitinib at doses of >10 mg BID, had ruxolitinib dose reduced to 10 mg BID.27 About a third of the 24 evaluable patients had a ≥35% reduction in spleen volume from baseline, and navitoclax also elicited a 20% improvement in TSS. Reductions in driver mutation allelic burden of >5% also were reported, as well as improvements in bone marrow fibrosis (BMF).
Table 2. Summary of the Key Ruxolitinib, Fedratinib, and Pacritinib Clinical Studies.2-6
| Trial | Study Design | Population | Response, % | Grade 3/4 AEs |
| Ruxolitinib | ||||
| COMFORT-I1 | Phase 3, randomized, double-blind, placebo-controlled Ruxolitinib: n=155 PBO: n=154 |
Patients ≥18 years with IPSS intermediate-2 or high-risk MF, platelet count ≥100×109/L, and splenomegaly |
|
|
| COMFORT-II2 | Phase 3, randomized, open-label Ruxolitinib vs BAT |
Patients ≥18 years with IPSS intermediate-2 or high-risk MF, platelet count ≥100×109/L, and splenomegaly |
|
|
| Fedratinib | ||||
| JAKARTA-13 | Phase 3, randomized, placebo-controlled, 3-arm study
|
Patients ≥18 years with IPSS intermediate-2 or high-risk MF, platelet count ≥50×109/L, and splenomegaly |
|
|
| Pacritinib | ||||
| PERSIST-14 | Phase 3, randomized, open-label
|
Eligible patients had intermediate of high-risk MF, PPV-MF, or PET-MF, no platelets cutoff, and splenomegaly |
|
|
| PERSIST-25 | Phase 3, randomized, open-label
|
Eligible patients had primary MF, PPV-MF, or PET-MF; ≤1 prior JAK2 inhibitors; and platelet counts ≤100K/µL |
|
|
AEs=adverse events; BAT=best available therapy; BID=twice daily; IPSS=International Prognostic Scoring System; MF=myelofibrosis; PPV-MF=post-polycythemia vera myelofibrosis; PET-MF=post-essential thrombocythemia myelofibrosis; QD=once daily; TSS=tumor symptom score
Back to content